Novel Li-F-H Compounds
A new CDAC paper from the groups at Buffalo, UIC, and LLNL reports predictions of new pressure-induced chemistry between LiF and hydrogen, with results that have implications for dynamic compression experiments.
Lithium fluoride is a common window in shock compression experiments, and pressure-induced chemical reactions between hydrogen and LiF windows in dynamic compression experiments near 300 GPa, could affect the interpretation of the results. Given the propensity for formation of stable and metastable ternary hydrides under pressure, crystal structure prediction techniques were applied to study the Li-F-H system to multimegabar pressures. Phase diagrams of the elemental (Li, H, and F) and binary (Li-H, H-F, and Li-F,) systems have been studied computationally corresponding to pressures up to 300 GPa, and these results have provided a well-established starting point for exploration of potential high pressure phases in the ternary system.
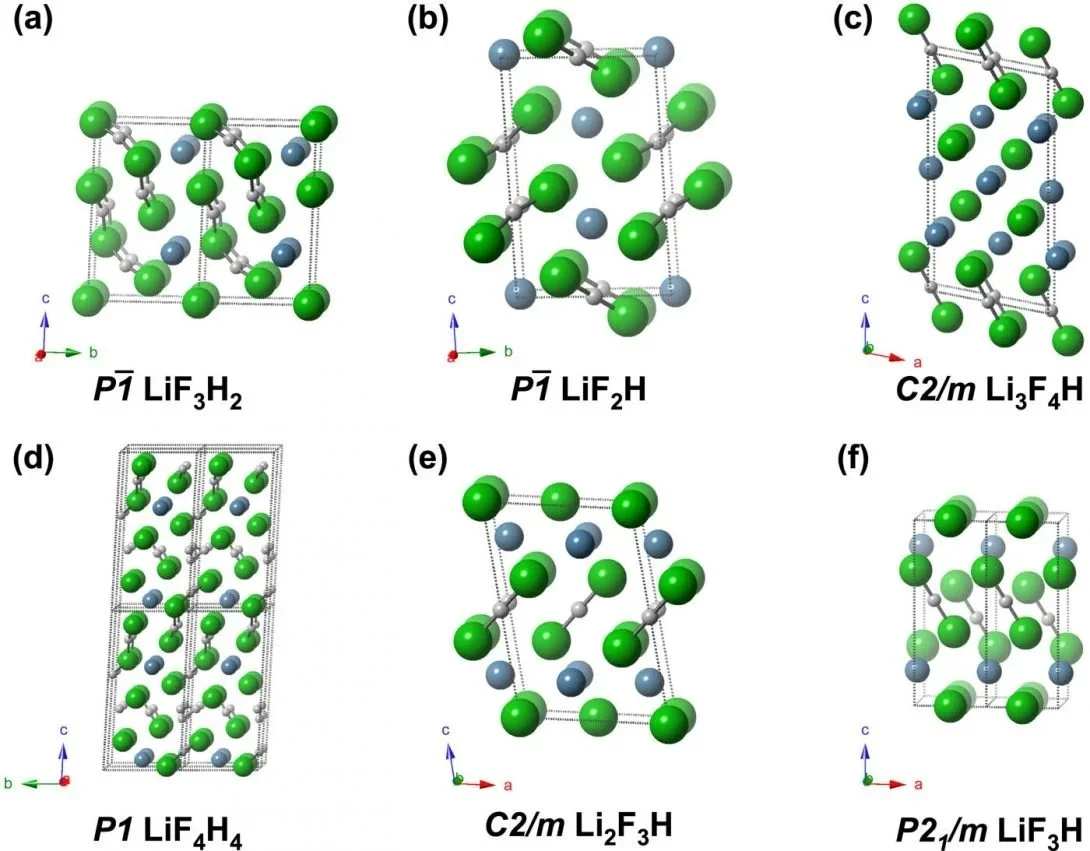
Evolutionary crystal structure prediction techniques were used evaluate ground state structures containing the elements Li, F, and H at elevated pressures. None of the structures found suggest compound formation in NIF experiments from the combination of LiF and hydrogen isotopes. A number of intriguing metastable phases, however, are predicted that could potentially be synthesized. Common structural motifs present in these phases include HnFn+1– anions of various lengths, and Li+ counter-cations. Most of these crystalline phases are predicted to be wide-gap insulators, with the exception of LiF3H, which calculations predict to be metallic and superconducting below 0.1 K. Li3F4H is found to be thermodynamically and dynamically stable at atmospheric pressure.
Exploratory calculations on the Li3F4H composition predict a structure that contains bent and asymmetric bifluoride anions, as well as infinite HF chains with nearly equal bond lengths, whose enthalpy lies 21.7 meV/atom above the convex hull. The fact that Li3F4H is predicted to be thermodynamically and dynamically stable at ambient pressure, suggests that other Li-F-H compounds with unique HnFn+1-type compositions could also be synthesized and stabilized at ambient conditions (Fig. 1).
These studies provide the basis for future work exploring the finite temperature stability of Li-F-H phases, with the inclusion of anharmonic effects, which are known to be important for light element systems, especially at high pressures.
Bi, T. , A. Shamp, T. Terpstra. R. J. Hemley and E. Zurek, The Li-F-H ternary system at high pressures. Journal of Chemical Physics 154, 124709 (2021).
Figure 1. Structures of boron polymorphs stable at 100 GPa. Left, a structure based on the alpha-Ga arrangment. Right, a structure based on the channel motif.