X-ray Diffraction and Equation of State of the C-S-H Room-Temperature Superconductor
A new paper from groups at UIC, Rochester, HPCAT and GSECARS reports an x-ray diffraction study of the recently discovered carbonaceous sulfur hydride (C–S–H) room-temperature superconductor. The study indicates that the structure of the C-S-H superconductor is derived from the structures of previously established van der Waals compounds found in the H2S–H2 and CH4–H2 systems.
Crystals of the superconducting phase were produced by a photochemical synthesis technique, and yielded a superconducting critical temperature (Tc) of 288 K at 267 GPa. For this work, x-ray diffraction patterns measured from 124 to 178 GPa (i.e. within the pressure range of the superconducting phase), are consistent with an orthorhombic structure derived from the Al2Cu-type determined for (H2S)2H2 and (CH4)2H2 but differ from those predicted and observed for the H3S system, which also exhibits a very high Tc at these pressures.
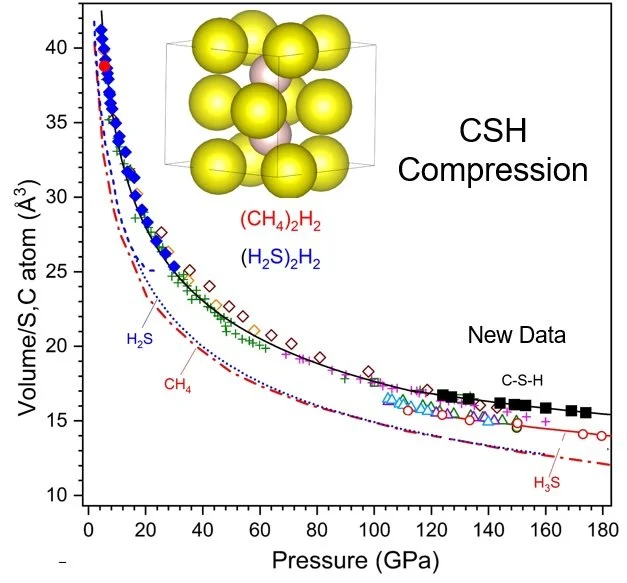
The formation and stability of the C–S–H compound may be understood in terms of the close similarity in effective volumes of the H2S and CH4 components (Fig. 1) Denser carbon-bearing S–H phases are predicted to form at higher pressures, and the current results provide a critical starting point for understanding the very high superconducting transition temperatures found in the C–S–H system at megabar pressures.
Lamichhane, A., et al., X-ray diffraction and equation of state of the C–S–H room-temperature superconductor. Journal of Chemical Physics 155, 114703 (2021).
Figure 1. Extended P-V equations of state for phases and phase components in the C-S-H system. New data from the present work are shown as black squares. Inset: Structure model showing the location of methae and and dihydrogen sulfide molecules (yellow spheres) and rotationally disordered (J = 0) hydrogen molecules.